I recently had the honor and pleasure of attending an event co-organized by the Outreach Network for Gene Drive Research at the European Parliament in Brussels, and delivering a presentation on our work at Target Malaria to develop new genetic tools to reduce malaria transmission in Africa.
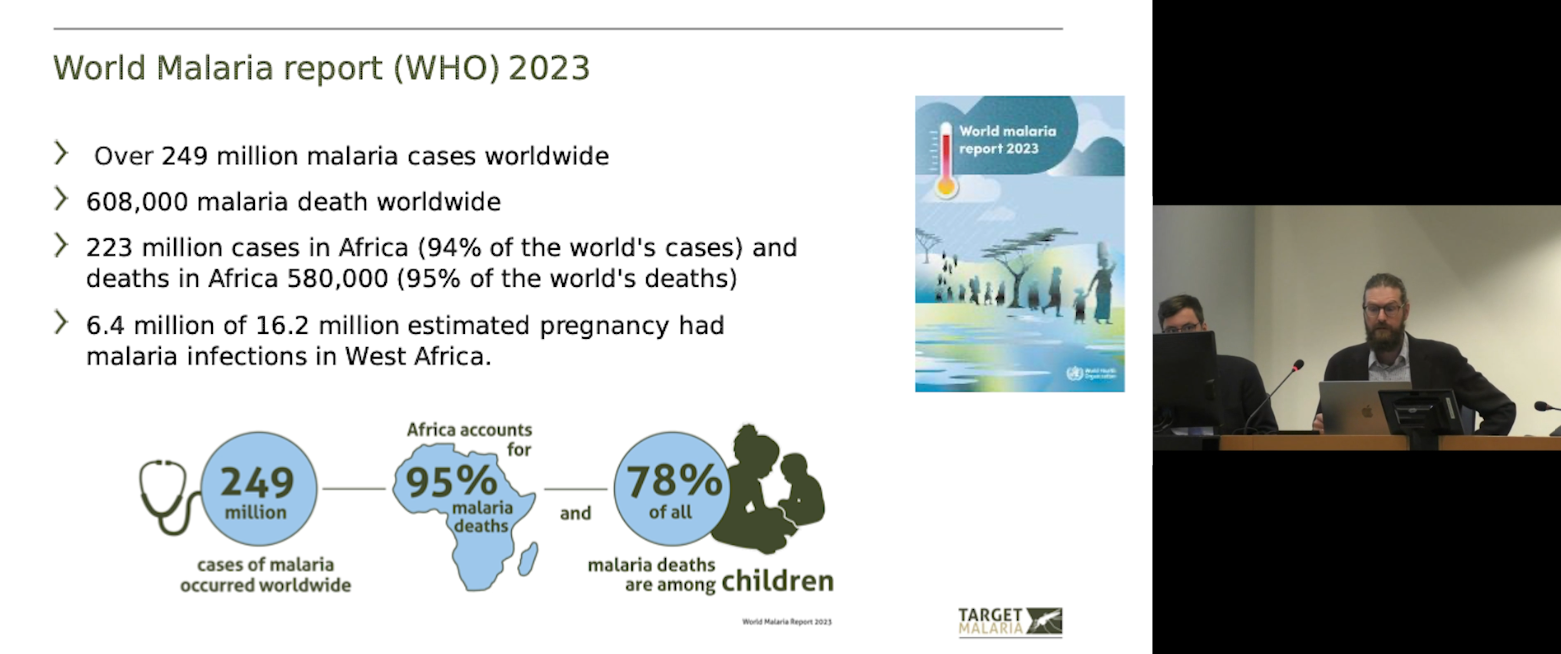
Alekos Simoni, Target Malaria, highlighting the global burden of malaria according to the WHO’s World Malaria Report 2023.
The event was an opportunity to discuss advancements in the development of gene drive technologies and governance implications related to these new tools. With climate change creating favorable conditions for the spread of disease-carrying mosquitoes, and challenges such as drug and insecticide resistance threatening the efficacy of current interventions, a global, coordinated response is needed to tackle vector-borne diseases. In this context, gene drive technologies could offer a promising addition to existing approaches.
MEP Charles Goerens opened the session by highlighting the global burden of vector-borne diseases, such as malaria and dengue, which cause over 700,000 deaths every year. He stressed the importance of innovation to address global health challenges and the critical role of the European Parliament in ensuring responsible oversight of new tools, such as gene drive technologies.
I opened my address by highlighting the devastating impact of malaria, a disease which continues to kill over 600,000 people every year, a majority of which are children under the age of 5 in Africa. I explained our goal at Target Malaria, which is to develop and share novel genetic technologies to reduce malaria transmission, and talked about what makes gene drive technologies a potentially promising addition to the malaria control toolbox – such as the fact that they are self-sustaining and could present a cost-effective approach to fight malaria. The approach we are focusing on at Target Malaria is called population suppression. It involves genetically modified mosquitoes carrying a modification able to impact their ability to reproduce. This would lead to a progressive reduction in the number of Anopheles gambiae mosquitoes — one of the main vectors of malaria in sub-Saharan Africa – and subsequentially to a reduction in malaria transmission. Our technology is currently in the laboratory phases, where it is being evaluated, and results are promising. We aim to progress towards potential field trials in the coming years, pending regulatory approvals from the relevant authorities.
My presentation was followed by contributions from several experts and policymakers. Florence Débarre, Centre National de la Recherche Scientifique (CNRS), discussed the crucial role that mathematical models play in gene drive research, and how they can help scientists understand and evaluate the potential outcomes and risks involved ahead of conducting work in the field. Yann Devos, European Food Safety Authority (EFSA), delved into EFSA’s role in providing guidance and ensuring that novel technologies meet safety and environmental standards. He explained that while the current EU guidance for genetically modified organisms is generally adequate, it may not fully address certain specific aspects related to gene drive modified insects. He highlighted the importance of a case-by-case risk assessment for gene drive technologies, as there are many different types of gene drive approaches, for many different uses and contexts.

Chair, presenters and participants of the session at the European Parliament in Brussels.
Next, Katherine Littler, World Health Organization (WHO), provided an overview of the global governance issues surrounding gene drive technologies, focusing on ethical, safety, and equity considerations. She pointed out that the WHO has developed a series of guidance documents, including the “WHO Guidance Framework for Testing Genetically Modified Mosquitoes“, which describes a phased pathway for the research and implementation of genetically modified mosquitoes and provides considerations for ensuring these technologies are developed and tested responsibly.
Werner Shenkel, Federal Office of Consumer Protection and Food Safety (BVL), Germany, provided insights into the role of international agreements, particularly the Convention on Biological Diversity (CBD), in shaping policies surrounding gene drive technologies. Finally, Her Excellency Madam Ambassador of Uganda to the EU and OACP, Mirjam Blaak Sow, talked about the heavy burden of malaria in Uganda, where the majority of the population is at risk, and highlighted the key role of African nations in shaping the research and development of these technologies. She gave the example of the Uganda Virus Research Institute (UVRI), Target Malaria’s partner institution in Uganda, which is leading vector control efforts in the country.
The event showcased the meaningful strides made to advance gene drive technologies across multiple fronts – scientific research, risk assessment, and governance, while underscoring the challenges that still lie ahead. It also highlighted the importance of a coordinated approach to help advance research on innovative technologies, and edge us closer to the elimination of vector-borne diseases.
