By John Connolly, Senior Regulatory Science Officer, Imperial College London, Target Malaria UK
& Dickson Lwetoijera, Principal Research Scientist, Ifakara Health Institute (IHI), Transmission Zero
Gene drive technologies offer a promising avenue to accelerate malaria elimination. Alongside existing tools, they could offer sustainable and cost-effective approaches to help eliminate malaria. As research on these technologies progresses – with several research groups having demonstrated the potential of gene drive approaches to control malaria mosquitoes in laboratory settings – researchers have also been considering how best these technologies could be evaluated in the field.
In a recently published paper in Malaria Journal, we outline how field evaluations of a self-sustaining or “low-threshold” gene drive for malaria control could take place. Unlike insecticide-based malaria vector control interventions, like bednets for example, low-threshold gene drive is expected to spread and persist indefinitely in target mosquito populations. This property means our gene drive approaches offer unique opportunity and strengths in malaria vector control. For example, they should require minimal numbers of mosquitoes in releases to achieve efficacy in the field or could be effective in controlling malaria without the need for changes in human behaviour.

Aayushi Sharma, Imperial College London, Transmission Zero, examining the malaria parasite. Photograph: Transmission Zero
Building on the WHO’s Guidance framework for testing genetically modified mosquitoes, we considered some of the key challenges, efficacy and risk questions, knowledge gaps, and potential solutions that would apply to initial field evaluations of gene drive mosquitoes. The publication describes key considerations for the design of field evaluations and is the product of a collaborative group of researchers, including three leading developers of gene drive technologies for malaria control, convened by the Foundation for the National Institutes of Health (FNIH).
Ultimately the decision on whether to allow field evaluations, and what would be an acceptable design, will rest with national authorities. The paper can hopefully feed into their reflections on this topic. It outlines several possible approaches, as well as many of the considerations that will need to be factored into the design of the possible evaluations and the choice of locations.
When considering field trials, the first question that one might ask is: what do we need to evaluate? A key aspect of the work described in the paper is the recognition that the use of gene drive for malaria control involves what we have called a “causal pathway” (see diagram below), and it is this pathway that would need to be tested to determine whether gene drive is effective for malaria vector control:
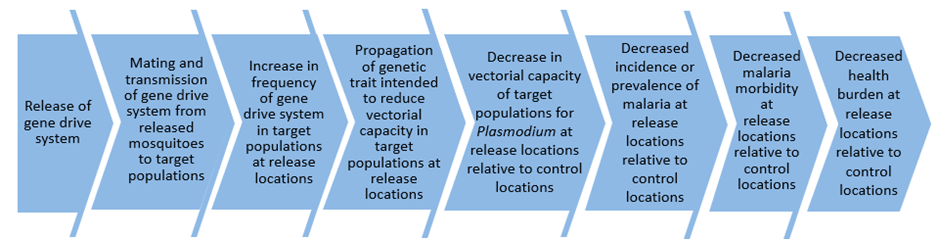
While there is a wealth of previous experience in testing new interventions like insecticide-treated bednets for malaria control in the field, this is not the case for gene drive where field evaluations have yet to take place.
In addition, while the expected spread and persistence of low-threshold gene drive is one of its key strengths, it also means there is more to consider for the design of the first field evaluations than for more conventional interventions. For example, ideally, the design of the first field evaluations might occur on a small scale and involve only one or two release locations and have only simple goals, perhaps testing early stages in the causal pathway, like measuring the rate of increase in the proportion of mosquitoes carrying the gene drive transgene. However, any subsequent and larger trials of gene drive that might evaluate the impact on malaria in the latter stages of the causal pathway could be affected by “spillover” effects from the earlier releases of our gene drive mosquitoes, as these continue to persist in the environment.

Anopheles gambiae mosquitoes in large cages at Polo d’Innovazione di Genomica,Genetica e Biologia (Polo GGB) research centre in Terni, Italy. Photograph: Target Malaria
Therefore, even before the first field evaluations can be designed, one needs to know already what those larger trials might look like, what their purpose is, and how they will measure impacts on malaria. Then, one can work backward to the first and simplest “pilot” trials to determine how they can best be designed and conducted to give the maximal useful information to inform the design of those larger, later trials. For example, a crucial aspect of an initial pilot trial of gene drive might be to determine the rate of spread of the gene drive transgene from release locations in one of the earlier steps in the causal pathway. Once this information is established in the field, it can then be used to determine what distances there might need to be between those initial gene drive release locations in pilot trials and release locations in later, more complex trials to evaluate the impact on malaria in later stages of the causal pathway.
Field evaluations are a crucial step in the responsible research and development of gene drive technologies. By providing real data and insights, these evaluations will be essential to help validate the effectiveness, safety, and practicality of this technology before implementation. The paper provides several potential solutions to some of the design complexities of initial gene drive field evaluations. We hope it helps researchers design entomological baseline studies and feeds into regulatory authorities’ reflections as they decide what they would consider to be an acceptable design for field evaluations.
Read the full paper here.
